Speed and Rhythm in i3’s 2G Brain Training Game
2G n-back training breaks up the input rhythms, forcing attention to continually reset its oscillations. This has 3 effects that should benefit working memory training:
- Training attentional flexibility. The unpredictable breaks in rhythm requires rapid ‘counter-phase’ attention switching. This trains the ability to disengage and shift attention – an essential skill in multi-tasking.
- Training processing speed. The faster presentation rate puts higher demands on your working memory and requires faster response times.
- Irregular stimulus presentation also prevents automatization of the attention system’s sensory processing. Working memory training benefits from preventing automatization when you can process on ‘automatic pilot’ mode.
The Science
Electrical activity in the brain can be recorded using EEG electrodes on the scalp. Different types of brain waves (electrical oscillations) have been linked to sleep, navigation, cognition, attention, and can help diagnose a wide range of disorders including autism, schizophrenia and epilepsy.
During standard dual n-back training, while our attention system keeps track of the updating letters and squares, sensory areas of the brain go into an electrical rhythm that matches the rhythm of the n-back letters and squares – for instance, every second. The n-back stimuli drive the cortex rhythmically (Lakatos et al., 2009).
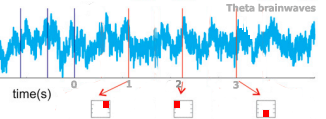
Adapted from Fig. 3, Lakatos et al. (2013)
The cortical brain rhythm helps process the stimuli, anticipating when the next sounds and squares will appear, and ‘binding’ the audio and visual information together in working memory.
How does this relate to real world cognition? Speech is rhythmic, our gestures are rhythmic, visual saccades (the moment to moment eye movements that we scan scenes or text with) are rhythmic. Our attention system works in these rhythms, resulting in periodic increases in excitability in anticipation of attended stimuli – making information processing more selective and efficient.
The speed and rhythm of the stimuli (letter sounds and square moves) in our 2G n-back can be systematically changed to increase speed and break the rhythm of the n-back stimuli, requiring rapid attention switching and demands on working memory.
The speed & variability setting is the most challenging of all the 2G options. Training with this option is for experienced n-back users who want maximum attentional demands!
 Cortical Rhythms of Attention – The Science
Cortical Rhythms of Attention – The Science
Selective attention
Working memory – our mental ‘workspace’ – depends on selective attention, a fundamental cognitive capacity. This allows the brain to enhance its coding of events in the world that are relevant while tuning out irrelevant or distracting events (Desimone & Duncan, 1995).
Selective attention is based on rhythmic brain waves, with the high excitability phase of the oscillation in phase with the rhythmic inputs (such as speech sounds, or visual fixations while scanning a scene). This results in the sensory responses to attended stimuli being predictively amplified while processing of stimuli ‘out of phase’ (in the troughs of the wave) are dampened. Attention to rhythmic streams in any one modality (e.g. visual) results in a synchronous ‘entrainment’ (tuning in) of neuronal activity across other modalities (e.g. audio), making it easier to integrate multi-modal information about the world around us (Lakatos et al., 2009; Bosman et al., 2009; Saleh et al., 2010; Besle et al., 2011; Lakatos et al., 2013).
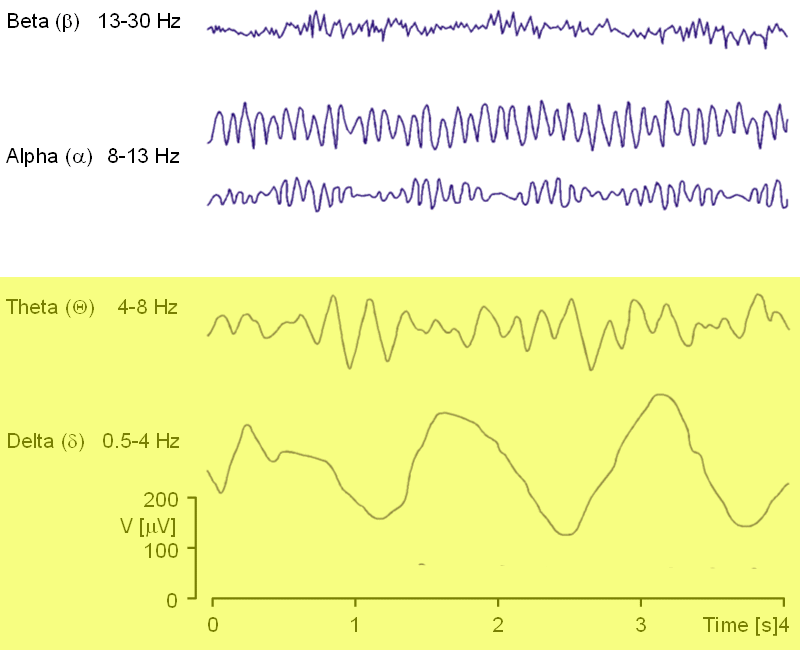
These attention-based rhythms occur in theta and delta bands 0.5 – 7 hz (cycles per second). Other rhythms are associated with other types of brain states. (Alpha waves, for instance, are associated with relaxed mental states such as those induced by meditation.)
Attention control & working memory
Phase resetting – to rapidly adapt to changing rhythms and ‘reset’ the brain waves – is under the control of our attention (Lakatos, 2009). Attention focus itself is part of our working memory system, and it is our working memory system that n-back training targets.
References
Besle, J., Schevon, C.A., Mehta, A.D., Lakatos, P., Goodman, R.R., McKhann, G.M., Emerson, R.G., and Schroeder, C.E. (2011). Tuning of the human neocortex to the temporal dynamics of attended events. J. Neurosci.31, 3176–3185[space]
Bosman, C.A., Womelsdorf, T., Desimone, R., and Fries, P. (2009). A microsaccadic rhythm modulates gamma-band synchronization and behavior. J. Neurosci.29, 9471–9480.[space]
Desimone, R., and Duncan, J. (1995). Neural mechanisms of selective visual attention. Annu. Rev. Neurosci.18, 193–222.[space]
Lakatos, P., Musacchia, G., O’Connel, M. N., Falchier, A. Y., Javitt, D. C., & Schroeder, C. E. (2013). The Spectrotemporal Filter Mechanism of Auditory Selective Attention. Neuron, 77(4), 750–761. doi:10.1016/j.neuron.2012.11.034[space]
Lakatos, P., O’Connell, M. N., Barczak, A., Mills, A., Javitt, D. C., & Schroeder, C. E. (2009). The Leading Sense: Supramodal Control of Neurophysiological Context by Attention. Neuron, 64(3), 419–430. doi:10.1016/j.neuron.2009.10.014[space]
Saleh, M., Reimer, J., Penn, R., Ojakangas, C.L., and Hatsopoulos, N.G. (2010). Fast and slow oscillations in human primary motor cortex predict oncoming behaviorally relevant cues. Neuron 65, 461–471.

